
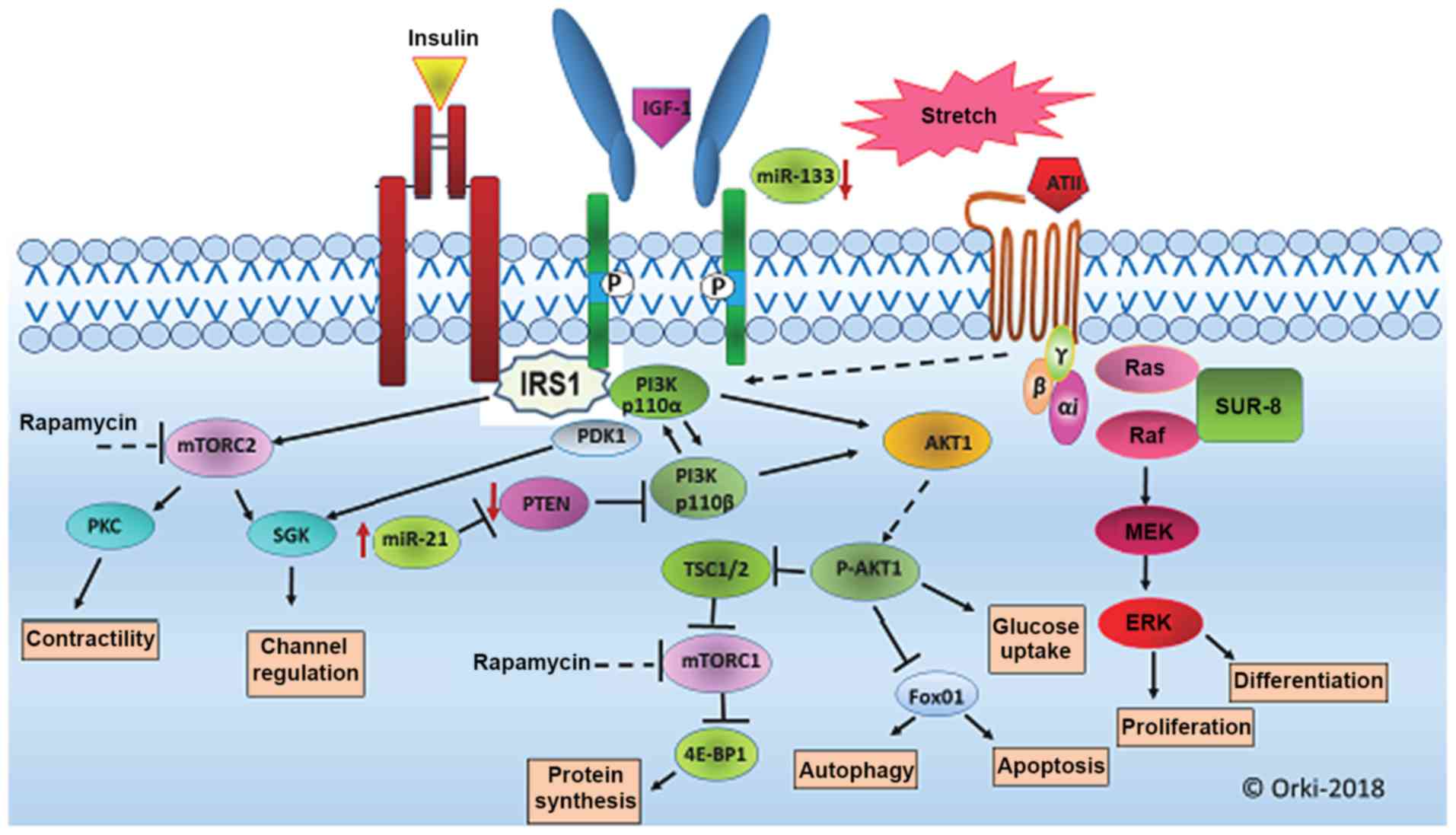
Impact of diabetes on cardiac structure and function: the strong heart study. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. Physiological and pathological cardiac hypertrophy. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Signaling effectors underlying pathologic growth and remodeling of the heart. Molecular basis of physiological heart growth: fundamental concepts and new players. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Epidemiology of heart failure with preserved ejection fraction. Heart failure with preserved ejection fraction. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. In this Review, we summarize the underlying molecular mechanisms of physiological and pathological hypertrophy, with a particular emphasis on the role of metabolic remodelling in both forms of cardiac hypertrophy, and we discuss how the current knowledge on cardiac hypertrophy can be applied to develop novel therapeutic strategies to prevent or reverse pathological hypertrophy.īraunwald, E. In the past decade, a growing number of studies have suggested that previously unrecognized mechanisms, including cellular metabolism, proliferation, non-coding RNAs, immune responses, translational regulation, and epigenetic modifications, positively or negatively regulate cardiac hypertrophy. Each form of hypertrophy is regulated by distinct cellular signalling pathways. Hypertrophy initially develops as an adaptive response to physiological and pathological stimuli, but pathological hypertrophy generally progresses to heart failure. There are two types of hypertrophy: physiological and pathological. Therefore, in the adult heart, instead of an increase in cardiomyocyte number, individual cardiomyocytes increase in size, and the heart develops hypertrophy to reduce ventricular wall stress and maintain function and efficiency in response to an increased workload. Cardiomyocytes exit the cell cycle and become terminally differentiated soon after birth.


 0 kommentar(er)
0 kommentar(er)
